Infant injuries can occur from hypoxia and more, but is refuted as a cause of newborn brain injury by naysayers. The clinical signs of neonatal encephalopathy are evaluated and ischemia is identified as pathogenic. The physiology of the third stage of labor and the transition from placental life support to the child’s life support systems by placental transfusion are detailed. Hypovolemia /hypovolemic shock is the origin of ischemic brain damage. Relationships between cord clamping, infant anemia and childhood neural and mental disorders are established. The timing of cord clamping at Cesarean, Vaginal, and Home birth and the consequent amounts of placental transfusion, are evaluated. Birth certificate worksheets for 235,215 Michigan live births were computer scanned for modes of delivery and signs of brain injury. Immediate clamping and cesarean birth incur the highest risk of brain injury; C.P. Midwife home birth with full placental transfusion avoids brain injury and promotes normal infant brain growth and development.
Key words: Cerebral palsy, Birth brain injury, Neonatal encephalopathy, Hypoxic Ischemic Encephalopathy, Immediate cord clamping , Delayed cord clamping, Blood pressure, Placental transfusion, Respiratory Distress Syndrome, Retraction Respiration, Central Venous Pressure, Central Nervous System, Multi Organ Failure, Multi Organ Dysfunction, Germinal Matrix, Intra Ventricular Hemorrhage, Fetal Heart Rate, Elective Cesarean Section, prostaglandin, Neonatal Intensive Care Unit, Intelligence Quotient
Survey of Related Factors in 235,215 Michigan Births
by
George Malcolm, MD, FACOG
Introduction
This paper elucidates the physiology of the third stage of labor. The documentation of injuries caused by disrupting that physiology with a cord clamp, and the long-term effects of those injuries is reviewed.
“Sick neonates are one of the most heavily [blood] transfused groups of patients in modern medicine.”[1] They frequently develop Cerebral Palsy (CP) that is usually attributed to birth hypoxia. CP is probably the most litigated human injury in the western world. The incidence of CP has remained fairly constant over decades, despite intensive measures to avoid and correct birth hypoxia. This strongly indicates that the pathogenesis of CP and birth brain injury (BBI) has been misdiagnosed.
Windle, in his experiments on asphyxiated primates,[2] noted that BBI never occurred in the primate’s natural state, indicating that the ultimate cause of primate CP was iatrogenic. Litigation is founded on medical error; hypoxia is the causal error. Human childbirth is physiological, a healthy function, not pathogenic, but it may have complications.
Modern human childbirth is “managed” obstetrics, designed to avoid complications and to preserve physiology – a normal, healthy outcome. However, management often intrudes on physiology, producing unintended consequences.
The review part of this paper defines the cord clamp as the causative pathogen of human CP, and also correlates a much larger scope of brain injury involving infant anemia and the child’s behavioral and cognitive functions (IQ). The investigative part shows that the pathogen is avoided in home births attended by Certified Professional Midwives, and that their method of practice prevents CP and all other aspects of BBI.
Current Opinion
ACOG’s (2006) Bulletin 348 [3] asserts that the brain lesions of cerebral palsy (CP) and neonatal encephalopathy (NE) originate from intra-partum hypoxia; more specifically, CP and NE “originate from four mandatory criteria that constitute an Acute Intrapartum Hypoxic Event as Sufficient to Cause Cerebral Palsy”:
A cord arterial pH <7.0 and base deficit of >=12 mmols/L;
Early onset of NE in an infant 34+ weeks gestation;
CP of spastic quadriplegic or dyskinetic type;
Exclusion of other etiologies e.g trauma, infection etc.;
This is a misconception and a contradiction, Cowan [4] states “there is no evidence that brain damage occurs before birth … data strongly suggests that events in the immediate perinatal period are most important in neonatal brain injury.” The TIMING of CP / NE / BBI is during AND AFTER birth. Cord arterial acidosis is generated over time before birth; the encephalopathy is generated over time after birth. ACOG’s mandatory causal criteria contradict each other time wise.
Bulletin 348[3] states: “Immediately after delivery the cord should be doubly clamped and divided…”
This is Immediate Cord Clamping (ICC.) Erasmus Darwin (1801) described this procedure as “injurious,” [5] Peltonen states, “the consequences [of ICC] may be fatal.” [6] Linderkamp stated: “ICC can cause hypovolemia, hypotension and anemia,” [7]
ACOG’s definition [3] excludes trauma and includes trauma in birth management.
The Origin of Brain Lesions
During and after birth, the brain is growing and developing, with many areas of active mitosis, and with incomplete “connectivity” between its various parts. The anatomical lesions of CP/NE/BBI originate in these areas of active growth and metabolism, and result in: Neuron necrosis (death of nerve tissue)
Impaired growth, development and connectivity of brain tissue.
In NE, areas of neuron necrosis are visualized on MRI / CAT / brain scans; the pathogen is also visualized – ISCHEMIA, deficient circulation. The necrosis originates and progresses in oxygenated neonates; it is generalized, not limited to a particular arterial supply area.
Hypoxia / Anoxia
Oxygen is necessary for aerobic respiration / metabolism, and is required for contraction (function) of cardiac muscle, (ADP -> ATP.) Oxygen is not necessary for survival of myocardium that can “live” on anaerobic energy if it is adequately supplied with glucose and nutrients, and if the metabolic waste products are removed – i.e. adequate circulation. Other tissues (including neural tissues) can survive similarly on anaerobic metabolism and adequate circulation; but again, function may be curtailed.
Tissue survival thus depends on CIRCULATION of nutrient blood, regardless of oxygenation. Glucose, not oxygen, is an essential nutrient.
CIRCULATION depends on the heart being supplied with oxygenated nutrient blood to generate a heartbeat and BLOOD PRESSURE. A pulsating cord in an apneic newborn confirms that the placenta is oxygenating the heart with nutrient blood, and that the heart is perfusing the placenta and the child. Oxygen is the essential nutrient for cardiac function.
Cardiac arrest (no BP, no circulation) results in necrosis of all tissues, however, those tissues (e.g. muscle) with stores of glycogen / glucose will survive on anaerobic metabolism longer than those without such stores. Brain tissue has no glycogen / glucose stores, and neuron necrosis (infarction) begins very rapidly after loss of circulation.
Markedly reduced circulation in areas of active metabolism will result in slowed metabolism, arrested growth and development, and eventually tissue necrosis as nutrients are exhausted and waste products of metabolism accumulate – regardless of oxygenation. (Oxygen may even speed exhaustion.)
In review, however, nearly every case of NE / CP has been subjected to some degree of hypoxia before and during birth; Cowan [4] defined neonatal encephalopathy on the following clinical criteria:
[A] Abnormal tone, [B] Feeding difficulties, [C] Lack of alertness, plus at least three of the following:
Late decelerations on the fetal monitor or meconium staining
Delayed respiration
Arterial cord pH less than 7.1
Apgar less than 7 at 5 minutes
Multi-organ failure.
The first three mandatory criteria indicate current CNS dysfunction / injury; the next four indicate a history of hypoxia; the fifth, multi-organ failure (MOF) indicates current, generalized ischemia. Delayed respiration contributes to an Apgar <7 and also indicates respiratory failure (MOF.) The lesions in the failing organs verify ischemia / hypovolemic shock, as the causative agent, not hypoxia. The affected organs are the heart, kidneys, gut, liver, lungs and the brain. [8, 9] Multi-organ dysfunction / failure is not caused by hypoxia as described by Hankins; [9] the organs continue to fail (to the point of necrosis) despite adequate neonatal oxygenation. Multi-Organ Dysfunctions: Heart (hypovolemia -> tachycardia -> hypotension -> hypovolemic shock): The hypotension / heart failure of MOF responds to vasopressors, intravenous fluids and especially to plasma volume expanders; it is not cured by oxygen.
Kidneys (Oliguria -> Anuria -> Acute Tubular Necrosis – ATN):
Ischemic ATN can be caused when the kidneys are not sufficiently perfused for a long period of time (i.e. renal artery stenosis) or during shock …. Oxygen does not correct oliguria, anuria in cases of shock.
Renal Cortical Necrosis RCN:
RCN may follow severe infection, severe dehydration, shock …
These lesions of RCN and ATN are, in effect, infarcts.
Gut (“Difficulty feeding” -> Necrotizing Entero-Colitis – NEC):
NEC is necrosis of the gut in the “watershed” region of blood supply of the superior and inferior mesenteric vessels – where these vessels anastomose and the blood supply is attenuated; NEC is a (often hemorrhagic) bowel infarct caused by ischemia of this region of the gut. NEC occurs in NE newborns and in preemies with germinal matrix infarction. In the adult, mesenteric artery stenosis can result in infarction of this region of the gut. Oxygenation does not prevent or cure NEC.
Liver (hypoglycemia):
The source of neonatal blood glucose is liver glycogen stores; deficient blood flow through the liver results in deficient conversion of glycogen to glucose, and lower blood glucose levels. Glucose is essential for aerobic and anaerobic respiration of neural tissues. Neonatal liver necrosis is rare; neonatal hypoglycemia is not rare. Elevated liver aminotransferase enzymes in NE are not lowered by oxygenation.[9]
The Lungs; Retraction -> Respiratory Distress Syndrome (RDS), Hyaline Membrane Disease (HMD) Bronchopulmonary Dysplasia (BPD).
These are terms that define [impending] lung infarct(s). The blood supply of the lungs is the bronchial arteries that arise from the descending aorta; they function in embryonic life through geriatric life, nourishing lung tissues. The pulmonary circulation, with a hydrostatic pressure in the pulmonary capillaries that is lower than the plasma colloid osmotic pressure, performs O2 and CO2 exchange, but it is not nutritive. In prolonged hypovolemic shock / ischemia, the lungs suffer the same consequences as the ischemic bowel and kidney, and necrosis of alveolar and bronchial tissue occurs. In adult hypovolemic / anaphylactic shock, hyaline membrane formation in lung tissue is diagnostic of “shock lung”. Oxygen does not cure HMD or RDS.
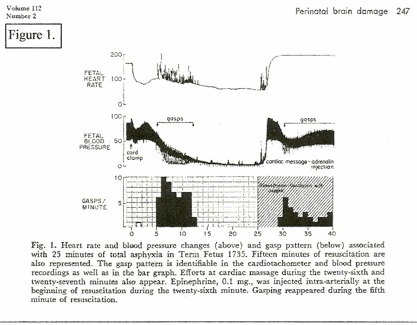
A constant feature in RDS is retraction respiration. (RR) This is illustrated as “Gasping” on (Figure 1.) and is “triggered” by low blood pressure; gasping can result in momentary reduction of diastolic blood pressure to zero – the equivalent of momentary cardiac arrest – resulting in marked tissue ischemia. RR pulls blood into the thorax and maintains the cardiac / coronary / pulmonary circulation at the expense of the systemic circulation – systemic ischemia. (Figure 1.)[10]
The Brain (Neonatal Encephalopathy NE / Hypoxic Ischemic Encepaphalopathy (HIE)):
Ischemia and progressive necrosis of the basal ganglia and the cerebral cortex can be followed in the oxygenated term neonate on MRI scan. In the preemie, the germinal matrix (GM) is extremely active metabolically, manufacturing spongioblasts and neuroblasts (mitosis ++) that migrate to form the cerebral cortex. The GM atrophies at 35 – 36 weeks gestation. Ischemia of the germinal matrix results in a hemorrhagic infarct; tissue necrosis in this very vascular organ results in bleeding into the cerebral ventricle (IVH) on re-perfusion. IVH occurs in oxygenated preemies.
Origin of the Hypovolemia/Ischemia
To define the original birth insult that caused the generalized ischemia, reviews of asphyxiated birth and physiological birth are needed as follows:
Primate Studies on Brain Damage Following Asphyxia
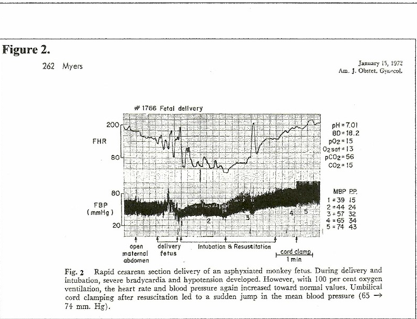
Studies on birth asphyxia in newborn primates [2, 10] produced brain damage and paralysis similar to human cerebral palsy; anoxia was induced by occluding the airway and clamping the cord immediately at cesarean birth. However, review of these records shows that the period of anoxia “causing” brain injury had to be long enough to cause heart failure and zero blood pressure (ischemia / cardiac arrest) for several minutes. (Figure 1.[10]) Resuscitation revived the heart and lungs, but did not repair ischemic neuron necrosis.
On the other hand, when fetal hypoxia was induced by asphyxiating the mother and the hypoxic neonate was resuscitated before the blood pressure had fallen to zero, the neonate recovered without injury. (Figure 2.[10]) In this case, significantly, the cord was clamped after the lungs were ventilated; the other primate (Figure 1.[10]) had the cord clamped immediately. (ICC) The timing of the cord clamp produced very different cardiovascular changes in each case:
ICC in the unventilated primate produced a precipitous drop in FHR and BP – hypoxia and hypovolemia / ischemia. (figure 1.) [6, 7]
Delayed cord clamping (DCC) in the ventilated primate produced increased BP and increased FHR – NO hypoxia or ischemia. (Fig. 2.) The “asphyxia studies” [2, 10] confirm ischemia as the causative pathogen in CP.
Physiological Birth – without a Cord Clamp
Man is the only mammal that clamps the umbilical cord of its offspring. Man is the only primate that produces young with cerebral palsy, except when primates’ cords are clamped immediately by men. [2] Erasmus Darwin identified the cord “tie” as pathogenic in 1801 [5, 11] He recognized that a large volume of blood was transferred from the placenta to the newborn in the moments after birth and stated that disruption of this transfer by tying the cord “too soon” was “very injurious” to the child – “the child is much weaker than it ought to be, a portion of the blood being left in the placenta, which ought to have been in the child.” Two centuries later, Cowan’s [4] mandatory clinical signs of NE – 1. abnormal tone, 2. difficulty feeding, 3. lack of awareness – eerily define Erasmus’s weak, hypovolemic, ischemic child. Even clamping the cord “too soon” is included in Cowan’s definition of NE: – a cord arterial pH can only be obtained by immediate cord clamping (ICC.) Cowan’s NE neonates with a cord arterial pH (any value) had a portion of their blood left in the placenta. In addition, ACOG’s definition of cerebral palsy [3] mandates ICC to obtain cord arterial blood – and the consequential obligatory blood loss. Comprehension of the pathogenic effects of cord clamping requires thorough knowledge of the physiological events that occur when a cord clamp is not used.
Placental Transfusion and Physiological Cord Closure
Figures 3, 4, and 5 are records of newborns’ weight changes from the time of birth; the weight change (gain) in grams is the volume of transfused blood in milliliters (mls). Pressures that force blood into the child are generated by:
Uterine contraction around the placenta
Maternal intra-abdominal pressure
Gravity (With the child positioned below the placenta.)
Pressures that force blood back into the placenta are:
Neonatal arterial blood pressure
Neonatal intra abdominal pressure
Gravity (With the child positioned above the placenta.)
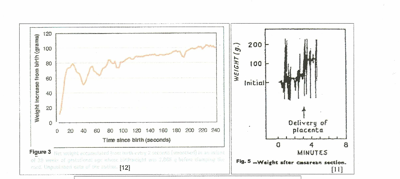
Visualized Placental Transfusion:
In figure 3, [12] 80 mls of blood were forced into the child within 20 seconds after birth by uterine contraction and maternal effort. In the next 20 seconds during uterine diastole, 30 mls were returned to the placenta by neonatal blood pressure. Thereafter, uterine contractions, (in a step-wise manner,) gravity and maternal intra-abdominal pressure gradually increased the volume transfused with little blood being returned to the placenta as the arteries closed.
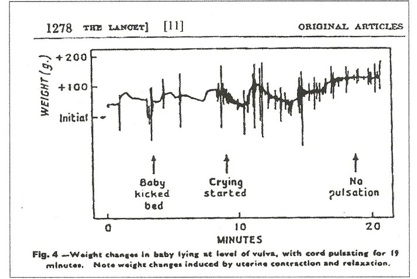
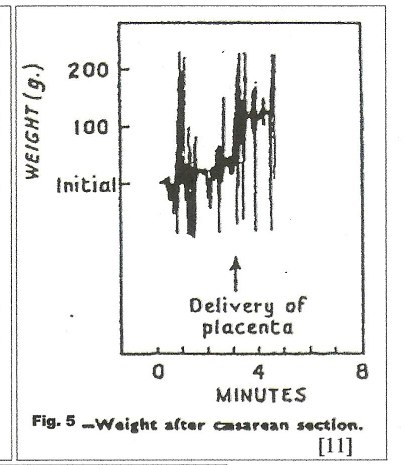
In figure 4, [11] no net transfusion occurred until after crying started, nine minutes after birth; the next contraction forced nearly 100 mls into the neonate in a period of 30 seconds; thereafter, contractions produced a “stepwise” increase in blood volume with no blood loss between contractions. Figure 5, a C-section, [11] shows a similar stepwise pattern with the final large and very rapid transfer of 100mls of blood as the uterus squeezes the placenta out of the uterus.
Visualized Power:
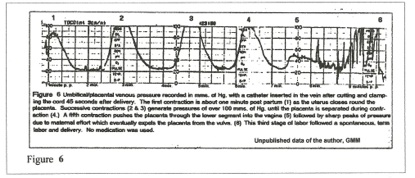
Figure 6 records umbilical venous pressures mms Hg) generated after birth by uterine contraction and maternal “pushing.” This record verifies the extreme hydrostatic pressure generated by the post-partum uterus in producing the rapid, massive transfer of blood volume through the umbilical vein that is illustrated in Figures 4,5,6, and in the later step-wise increase in blood volume. The baseline of about 20 mms Hg pressure is the maternal intra-abdominal pressure that is maintained until the placenta enters the atmosphere.
The total blood volume of the average term physiological neonate (delayed clamping) is about 300 – 350 mls. Of this total, 100 – 150 mls are usually transfused after the child is totally delivered. The forces of transfusion are also present during normal vaginal delivery, and those delivered parts of the child are filled with blood as they enter the atmosphere. (Note the engorged blue-black head of shoulder dystocia.) Overall, the child’s blood volume nearly doubles in the change from fetus to neonate during physiological birth.
At elective cesarean section (ECS) without labor, the forces effecting PT are absent, and if the child is above the placenta, reverse gravity transfusion may occur into the placenta – blood loss. With the usual ICC procedure, the ECS neonate may lose half or more of its natural blood volume. ECS babies are much more prone to respiratory problems than vaginal births, [34, 35, 36] death from persistent fetal circulation may occur.
Hemodynamics of Placental Transfusion in the Neonate
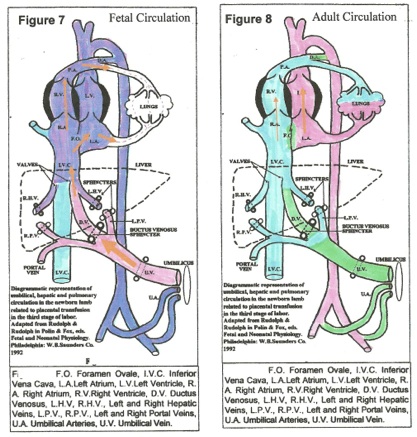
All studies on placental transfusion (PT) at spontaneous vaginal delivery confirm a very rapid transfer of 100+ mls. of blood into the child,[6, 7, 11,12, 13] under high pressure through the umbilical vein, ductus venosus and vena cava, resulting in distention of the heart chambers, the great vessels AND THE LUNGS. (Figures 7, 8)[6] Before birth there is minimal blood flow through the pulmonary vessels; the pulmonary arterioles are constricted and other pulmonary vessels are relatively collapsed, not turgid. PT (at 100+mms Hg pressure) forces blood through the foramen ovale, the pulmonary vein and artery, engorging the lungs and establishing the pulmonary circulation. [14] During uterine diastole, pressure in the right atrium falls and the foramen ovale closes permanently, establishing the adult circulation. (Figure 8)[ 6]
Distension of the pulmonary circuit with blood was researched by Jaykka [14]. Forced injection of blood into the pulmonary vessels of unexpanded fetal lungs caused “erection” of lung tissues with air entering the bronchi and alveoli. [14] Forceful placental transfusion ventilates the lungs, establishes pulmonary respiration and the adult circulation. [6] The high colloid osmotic pressure of blood in pulmonary capillaries absorbs amniotic fluid from alveoli, drying out the lungs. The PT imposes
the “first breath” on the neonate.
Physiological Cord Vessel Closure
After lung ventilation, increased oxygen tension in arterial blood causes PROSTAGLANDIN (PG) release from the umbilical artery walls into the fetal bloodstream. In response, the umbilical arteries constrict, and then close. Circulating PG also closes the Ductus Arteriosus and the Ductus Venosus, completing the adult circulation. [15]
The umbilical vein closes reflexively with (intra-abdominal) sphincters, [figures. 7, 8] probably governed by pressor receptors in the atria, producing the “stepwise” pattern of transfusion that is described in most studies. In the “steps,” the transfusion generated by uterine contraction is abruptly halted and no blood flows out – sphincter closure — no weight loss. During uterine diastole, central venous pressure (CVP) falls, the sphincter relaxes a little (without blood loss) and the next contraction forces in more transfusion that again closes the sphincter, akin to a pressure valve. (figure 4) Permanent vein closure occurs when a stable, optimal CVP and an optimal blood volume are attained.
Human Studies
Recent reviews on delayed cord clamping (DCC) in term infants have confirmed the beneficial and benign nature of the procedure [13] and the beneficial effect of DCC in preemies. [16, 17, 18, 19] No “double blind” study has been done comparing ICC to NO Cord Clamp in compromised / asphyxiated neonates. In Cowan’s study, [4] all the neonates were hypoxic during birth (criteria 1 – 4) and most, if not all had ICC – cord arterial pH.
Many publications over the past 20+ years have attempted to establish a correlation between cerebral palsy and a cord arterial pH value as a measure of the “hypoxic insult.” Despite ACOG, [3] >7.0 and Cowan [4] >7.1 no numerical correlation between brain damage and cord arterial pH has been established. There is a 100% correlation in these studies between CP and ICC, because every encephalopathic / CP child was subjected to ICC to obtain the cord arterial pH.
However, ICC on a normal birth does not result in NE / CP; [6] the elective c-section ICC child that receives no PT may be compromised, but seldom to the extent of CP. The combination of intra-partum hypoxia and ICC appears to be very pathogenic for CP / NE.
The Tourniquet Effect of Intra Partum Hypoxia:
Intra-partum hypoxia becomes manifest with signs of “fetal distress” (bradycardia, late decelerations, meconium staining, and a low scalp pH – some of Cowan’s criteria for NE.) The cause of this “distress,” in nearly every case, is compression of the umbilical cord – a tight nuchal cord, a prolapsed cord, a true knot in the cord, oligo-hydramnios – any circumstance that impedes the cushioning effect of adequate amniotic fluid surrounding the cord in the circuit between the placenta and the umbilicus.
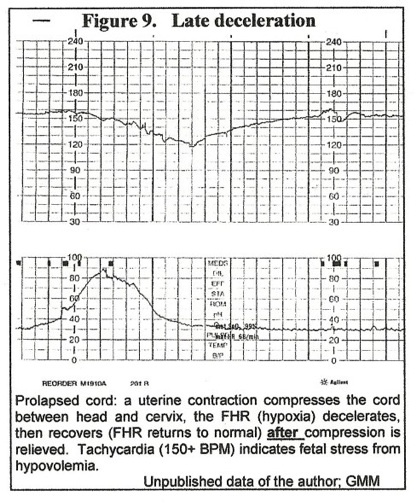
The initial result of cord compression is decreased blood flow in the umbilical vein – the “compressing force” decreases the diameter of the “low-pressure” vein much more than the diameter of “high pressure” arteries. Decreased venous supply of umbilical blood to the fetus means decreased oxygen supply – hypoxia – resulting in bradycardia. (Figure 1.) In the case of a prolapsed cord, when a uterine contraction compresses the cord between head and cervix, a “late” deceleration of the FHR (hypoxia) occurs that recovers (FHR returns to normal) after uterine diastole as cord vein compression is relieved. (Figure 9.)
With persistent cord compression, the DISTRIBUTION of blood volume between the fetus and the placenta does NOT return to normal. Persistent decreased blood volume return to the fetus means progressive engorgement of the placenta and increasing hypovolemia of the fetus – that becomes increasingly hypoxic (
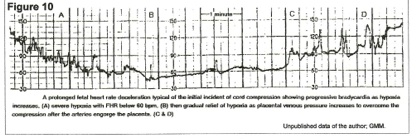
Interpretation of Figure 10 – venous tourniquet effect.
Occult cord prolapse occluded the cord vein at the beginning of the tracing, and between points A and B, the fetus used up circulating oxygen and became virtually anoxic from point B to point C. However, from A to C, the arteries were engorging the placenta and pressure in the vein was rising until, at point C, venous pressure overcame the compression and oxygenated blood began to flow back into the fetus – increase in fetal pulse rate. By the end of the tracing, oxygenation (FHR) was adequate, but there had been a massive blood volume shift from fetus to placenta over 9 to 10 minutes to accomplish this.
The patient was in early term labor (Figure 10,) and this episode was not noticed by attendants. 30 minutes later, late decelerations were noted (figure 9) and cesarean section revealed an occult prolapse of the cord.
The child was pale at birth but was allowed to breathe spontaneously, the cord was not clamped; maternal intravenous oxytocin was given to effect uterine contraction and placental transfusion. (figures 5, 6) The five minute Apgar score was ten. There was no meconium staining of the fluid. Umbilical arterial cord blood was not sampled as the cord vessels closed physiologically and were empty at the time of cord clamping. This child was subjected to five or six minutes of intense hypoxia / anoxia with closure of the umbilical vein, but had NO brain damage. Hypoxia is not a pathogen as long as the heart is beating and generating blood pressure.
The relatively benign outcome of this case was the result of fairly early recognition and correction (C-section) of cord compression and the correction of hypo-volemia by DCC and placental transfusion. If ICC had been employed at birth, the outcome could have been NE.
Chronic cord compression:
When cord compression persists for a prolonged period, (persistent late decelerations) the high pressure in the placental capillaries can result in fluid loss into the maternal circulation and fetal dehydration; fetal / placental blood volume is depleted and generalized fetal / placental vasoconstriction intensifies. Ischemic hypoxic tissues result in metabolic acidosis. In the extreme, the child is born “dish-rag” limp, ashen grey in color with dark blue patches of cyanosis, and with narrowed, pulsating cord vessels, bradycardia, and metabolic acidosis. ICC in this case will result in NE / CP / and possibly demise. This kind of case populates ACOG’s definition of “an Acute Intrapartum Hypoxic Event as Sufficient to Cause Cerebral Palsy.” (The event is chronic, not acute.) The primary pathogen in such a case is hypovolemia, not hypoxia. [3, 9, 20]
The key to successful resuscitation in such an extreme case is to recognize hypovolemic shock as the primary pathogen, and placental function as the principal rational resuscitator. If the heart is beating, the placenta is functioning, DO NOT CLAMP THE CORD. Release a nuchal cord; [somersault 21] maintain placental function. Neonatal oxygenated blood volume will be augmented; re-hydration and correction of acidosis will occur at the placental interface while cord pulsation – that may persist for many minutes – is present. [Figure 4.] The cord should be kept warm and moist and the lungs should not be ventilated – oxygenated blood closes the umbilical arteries and ends placental function. [15] If the heart rate is increasing or is above 100 bpm, the placenta is oxygenating the child adequately and is rectifying other metabolic accidents – acidosis, dehydration and hypoglycemia undergo correction with placental function. If the uterus is allowed to deliver the placenta with the cord intact, full PT should restore the child to normal newborn status. The cord must remain intact until respiration is established.
Placental Abruption
Intra-partum separation of the placenta from the uterine wall results in fetal hypoxia. The condition is rare compared to cord compression and the hypoxia (and bradycardia) is not accompanied by initial fetal hypovolemia. As the hypoxia intensifies, however, the fetus responds by generalized vaso-constriction, shifting blood flow to the heart, brain and placenta. At birth the cord should not be clamped until the placenta is delivered. In larger abruptions, due to ante-natal disruption of all placental functions (nutrition, electrolyte / acid-base balance, excretion as well as respiration,) neonatal recovery is usually much slower (and often less successful) than in the cord compressed newborn that uses these functions in resuscitation – without clamping. The additional complications of maternal defibrination (DIC) multiply the hazards of blood loss to the mother as well a the newborn.
The routine neonatal treatment of the “asphyxiated” neonate – ICC and removal to ventilation is disastrous. Peltonen [6] describes tumultuous cardio-vascular changes when the cord is clamped before the first breath. Sudden bradycardia and hypotension after ICC (figure 1) is due to anoxia and loss of venous filling of the ventricles, leading to cardiac asystole for several cycles.[6] Recovery usually occurs when blood flows through the lungs:
“Although the normal infant survives without harm, [with clamping before the first breath] under certain unfavorable conditions, the consequences may be fatal.” [6]
The severely asphyxiated / hypovolemic / acidotic child has minimal peripheral circulation, and ICC virtually stops venous return to the heart. Ventilation relaxes the pulmonary arterioles; if blood from the systemic circulation fills the lungs, systemic blood pressure will collapse resulting in shock and multi-organ failure, NE and possible fatality. To enable and sustain pulmonary AND systemic circulation after birth, the cord must remain intact until respiration is well established. [5]
In the less extreme cases of fetal distress, (Figure 10) restoration of the physiological parameters (Apgar 10) is usually very rapid when a cord clamp is not used. The whole protocol of neonatal resuscitation for the “at risk” term child or for the preemie [17] should be revised to allow for placental function and for physiology to continue through the third stage of labor at every birth. The cord must not be clamped. “There is good reason in cases of resuscitation to keep the placental circulation intact”.[6]
Retraction Respiration – “Gasping.”
A significant symptom recorded on the asphyxiated primate (Figure 1) and often present on the ICC / depressed human neonate is “GASPING” – retraction respiration. (RR) Gasping started (Figure 1.) at about 50 mms Hg systolic BP, and lasted until the BP was zero (CNS collapse.) Spikes of increased pulse rate are recorded during gasping with sudden drops of diastolic BP to zero. Gasping is a reflex response to low BP, low cardiac output and low Central Venous Pressure (CVP). Very low intra-thoracic pressure (gasps) pulls venous blood into the heart and lungs, filling the ventricles and briefly increasing the pulse rate; arterial blood is also pulled back into the thoracic aorta, briefly dropping systemic diastolic pressure to zero. (figure 1.) In the immediately clamped, hypoxic neonate, already hypovolemic and hypotensive, frequent “gasps” will intermittently lower carotid artery pressure resulting “tidal” carotid blood flow and minimal brain circulation – ischemia and neuron necrosis, NE. Retraction respiration is a major factor in ischemic brain damage and MOF. [8, 22]
ANEMIA
On admission to an NICU, the neonate is usually assessed with a SNAP II – Score of Neonatal Acute Physiology – [23] higher scores indicating the severity of “illness.” The neonatology term for a newborn fitting Cowan’s definition of NE is “sick neonate.” “Sick neonates are one of the most heavily transfused groups of patients in modern medicine.” [1] The indication for these transfusions (of RBC’s only) is marked anemia – which eventually results from dilution of the neonate’s remaining red cell mass after blood loss by ICC. The anemia becomes evident a week or more after birth, long after hypovolemia, ischemia and MOF have created injury. The gasping, hypotension and oliguria of the sick neonate should be corrected immediately by whole blood transfusion, not by CPAP, vasopressors and IV fluids:
Immediate restoration of functional blood volume corrects ischemia and prevents infarction. Infant anemia indicates prolonged neonatal hypovolemia / ischemia and possible infarction / brain injury.
Hidden and Delayed Neural / Brain Defects:
ACOG’s definition of the cause of cerebral palsy [3] mandates specific motor disabilities – spastic or dyskinetic palsy – that are not clinically apparent in the neonate, even though clinical central nervous system injury may be evident. [9] The specific lesions of CP are located in the forebrain basal nuclei. The definition [3] also mandates moderate to severe neonatal encephalopathy (NE) indicating widespread injury of grey matter, including the cerebral cortex.
Thus CP / NE may be associated with a wide variety of brain dysfunctions depending on the location and extent of the prevalent injury to grey matter. General loss of cortex can affect cognition, (IQ) with massive loss of cortex resulting in imbecility. In preemies, damage to the germinal matrix (which “manufactures” the cortex) can result in neural and mental deficiency [24] Injury to specific cortical areas may affect specific functions – e.g. occipital lobes – sight, temporal lobe – speech, hearing memory.
“Minor” degrees of brain injury / dysfunction inflicted by local ischemia will not be clinically apparent in the neonate, and may not appear until childhood [23, 25, 26, 27.] or the young adult. [25, 26, 27, 30] In areas of active neural growth, ischemia can delay or prevent development and connectivity; a defined anatomical infarct may not be incurred. Windle described behavioral (sight memory) dysfunction in adult monkeys that had recovered from “asphyxia induced” paralysis at birth. [45,46]
Long Lasting Neural and Behavioral Effects of Infant Iron Deficiency
Lozoff et al [28] published a comprehensive review on this subject in 2006 confirming that infant iron deficiency / anemia correlated with, and even predicted [29] poorer cognitive, motor and socio-emotional function from pre-school age to adolescence. That correlation is not necessarily a cause-and-effect relationship between infant anemia and brain dysfunction as “most studies [on correction of the infant iron deficiency] show that differences in development and behavior persist … even after a full course of iron therapy.” However, the behavioral / neural defects and the infant iron deficiency could have a common pathogen, confirming a valid relationship.
One of the reviewed studies [30] using the Women, Infant and Children program(WIC) compared grade school IQ for Special Education admission to infant hemoglobin levels; the IQ (at age ten years) decreased by a factor of 1.28 for each lower unit of hemoglobin (infant.) The infant hemoglobin was taken at the first post partum visit (WIC) and if it were low, the infant would have been given supplemental iron. Anemic pregnant mothers in the WIC program are treated with iron for anemia – iron deficiency in the WIC infants is NOT nutritional in origin.
Lozoff [28] states: “Perinatal iron deficiency has received little attention, due in large part to previous thinking that infants are protected from maternal iron deficiency…” [31] Normal neonates are protected from iron deficiency – if the cord is not clamped immediately. [13, 32]
In an extensive review (#=1912 neonates) of early (ICC) and late cord clamping, (DCC) [13] clamping delayed for 2 minutes or more resulted in higher neonatal hematocrit levels and reduced the risk of anemia and infant iron deficiency:
“Perhaps the most important finding was that the beneficial effects of late cord clamping appears to extend beyond the early neonatal period. Our meta-analysis estimated a significant 47% reduction in risk of anemia and 33% reduction in risk of having deficient iron stores at ages 2 to 3 months with late clamping.” [13]
The obscure origin of infant iron deficiency can be thus identified with the current and widespread obstetrical habit of ICC at birth. Infant iron deficiency (and thus lowered IQ) can be largely PREVENTED by delayed clamping (DCC.) The ultimate in delayed clamping is physiological cord closure that produces a MAXIMAL, OPTIMAL blood volume.
Special Education (SE)
SE classes [30] harbor multiple behavioral and developmental diagnoses – attention deficit disorder (ADD), learning disorders, hyperactivity, aggression, Asperger’s, and autism / Autistic Spectrum Disorder, ASD. The common denominator for all the above SE qualifying diagnoses is mental retardation – low IQ, and the lower the IQ, the more severe is the disability. As seen in the WIC study, [30] the lower the infant hemoglobin (anemia) is, the lower is the grade school IQ. One “reason” for the autism epidemic is that many low IQ children have speech / communication difficulties and are labeled “autistic,” as they then qualify for Medicaid assistance. The autism epidemic is a “low IQ” epidemic, and a very serous social problem.
The low infant hemoglobin / iron stores reflect the blood volume deficiency in the neonate after ICC, – the lower the infant hemoglobin, the more severe was the immediate neonatal hypovolemia and brain ischemia immediately after birth. If these correlations are valid, the types of managed childbirth that engender ICC (no PT), DCC some PT, or full PT (physiology) should correlate with the respective neonatal outcomes, and should influence the ultimate IQ’s of those children.
Cesarean section, especially elective (ECS) usually entails ICC and little or no placental transfusion. Thus ECS infants should be prone to infant anemia and lower IQ’s. A large Australian study [33] found an increased incidence of autism in ECS children. ECS neonates are prone to respiratory distress (a MOF related to blood loss.) [25, 33. 34, 35, 36]
Complicated “at risk” births – C-section, prematurity, fetal distress, twins, etc. that anticipate newborn resuscitation – are usually subjected to the routine procedure of ICC and removal to a resuscitation table. These births result in an excess of ASD / SE children. [25, 26, 27, 33, 37]
A recent large study on resuscitation at birth, and cognition at 8 years of age, [38] showed that infants who were resuscitated had increased risk of a low IQ score. Resuscitation entails ICC and removal to a resuscitation table; it is usually performed on a neonate with delayed respiration – not breathing – a MOD. Peltonen advises that clamping before the first breath “should be avoided” as in some cases, ICC is possibly “fatal.” [6] The authors of this study [38] associate resuscitation with delayed respiration and hypoxia / ischemia, but they did not identify ICC as a cause of ischemic damage; hypoxia is the “deemed” pathogen.
The Enigma of Autism
Autism is a disorder of speech communication, but because of its multiple symptoms and degrees of disability, the term “autistic spectrum disorder” has been adopted. A characteristic of the severely disabled autistic child is echolalia – “parrot speech” – the child perfectly repeats
words that it hears, but when asked “What did you say?” the reply is “What did you say.” The sensory and motor functions of hearing and speech are intact, but sound memory is impaired; sounds are shunted between hearing and speech without memory, without recall, and without cognition; even minor degrees of impaired communication lead to behavioral disorders and social isolation.
Without words or the meaning of sounds, thinking and learning are difficult. IQ evaluates the degree of disability. Sound / hearing memory is located in the cortex of the temporal lobes of the brain. The “autism defect” appears to be one of connectivity between the auditory and speech pathways of the brain and the temporal cortex. If that “connectivity” is being developed and constructed at the time of birth, birth ischemia may interfere with or arrest that development; however, ischemia may not be the only factor.
The placental transfusion is rich in stem cells that are the “building blocks” of all tissues, especially blood vessels and the immune system. Leaving a large supply of stem cells in the placenta could interfere with vascular brain growth and development.[12] The germinal matrix is very vascular.
The enigma arises from the fact that many more boys than girls are autistic, males exceeding females >2:1. The X chromosome appears to be protective against ASD birth brain damage – girls having XX against the boys XY. However, in mature tissue, one X in the female is sequestered as a Barr body, and inactivated, equalizing the “X” effect in mature sexes. On the other hand, if an autism “trait” were on the Y chromosome, girls would NEVER be autistic. They are.
Sugie [37] enumerated different birth complications with the numbers of autistic boys and girls. Asphyxia, fetal distress and respiratory distress produced exactly twice as many ASD boys as ASD girls. In the “post term” group, boys outnumbered girls 6:1. Post term girls appear to be almost immune to “birth brain injury” ASD.
Ischemic injury occurs in GROWING brain tissue, not in matured areas. Growth means mitosis, and during mitosis the X chromosome is restored to activity from its Barr body. If the active X chromosome influences the rate of brain maturation, at any gestational age, girls would average twice as much matured brain tissue as boys, and half as much growing tissue available for injury. In the WIC [30] study, “males were 2.17 times more likely … to be retarded than females. Anemic low birth weight and very low birth weight children were 2.5 and 4.48 times more likely to be retarded than normal children.” LBW and VLBW have actively growing, immature cerebral cortexes, with girls having twice as much matured brain tissue as boys, and half as much vulnerable brain tissue. But these girls are not immune to IQ damage.
If girls mature the “ASD area” completely by term, (no growing tissue) while the boys are still actively growing the “ASD area,” post-term ASD / ICC boys should greatly outnumber post-term ASD/ICC girls – as in Sugie.[37] Girls mature puberty earlier than boys do.
The incidence of neural, mental and behavioral disorders / mental retardation / autism is currently about 1 per 100 live births or less. Many of these children arrive in SE with an “autism” label attached, and some may have overt signs of motor impairment, CP. However, not all CP or autistic children are mentally retarded, but a good cognitive ability does not always compensate for the social and behavioral disability. Disruption of PT by a cord clamp is closely associated with an epidemic of behavioral and mental childhood disorders.
Proof of a causal relationship should be evident in a large cohort of children who were delivered without having their cords clamped.
The Consequences of Third Stage Physiology and Patho-physiology:
The magnitude of the potential blood loss caused by clamping the PT in the placenta is illustrated by Professor Peltonen [6]:
“If the problem were magnified to adult proportions, the amount of transferred blood would correspond to 1500-2500 mI. of blood poured into the circulatory system within a period of seconds or minutes. This being the case, we can say with certainty that the transfer of blood from the placenta into the newborn organism is a major and significant hemodynamic event, taking place at or immediately after birth. The accession — or its absence — of such a large quantity of blood into the newborn organism must play an important physiologic or pathophysiologic role in the infant.”
Peltonen poses the situation of an adult losing 2.0 liters of blood within a minute; the consequences are hypovolemic shock and demise. The newborn usually does not die from a similar event, (ICC,) but its organs are vulnerable to ischemic injury. The PT is physiology; absence of PT is pathogenic.
Definition of physiological birth requires documentation of multiple indicators of health in many neonates born without a cord clamp.
Definition of pathology resulting from absence of PT requires documentation of multiple indictors of illness in many neonates born with cords clamped before physiological cord closure occurred.
Cerebral Palsy, Neonatal Encephalopathy, Hypoxic Ischemic Encephalopathy, Mental retardation, Neural and Behavioral Disorders Autistic Spectrum Disorders
Brain ischemia causes the above brain injuries; ischemia results from blood loss when a large portion of the child’s normal blood volume is clamped in the placenta. The severity of the injury depends on the degree of hypovolemia. In the extreme, it may result in hypovolemic shock with overt infarction of multiple organs as well as the brain. With smaller blood loss, occult injury may consist of derangement of growth and development of neural tissues that may not be detectable until childhood or early adulthood. However, the blood loss is detectable in early infancy as anemia or low ferritin levels, and may also be EVIDENT TO SOME EXTENT IN THE NEONATE’S VITAL SIGNS IMMEDIATELY AFTER BIRTH.
The time of cord clamping is never recorded on the medical record, and the volume of blood left in the placenta is not recorded. There are multiple studies on early and delayed clamping, but there are none on no clamping – physiology.
Dozens of physiological third stages of labor occur every day across North America. Home births conducted by Certified Professional Midwives ((CPM) routinely have the cord clamped and cut after the placenta is delivered. Those births are documented on a birth certificate worksheet [figures 11, 12.] as Home births, the attendant being a CPM. A detailed description of the neonate and its condition (the outcome of third stage physiology) is also documented. Hospital births are also recorded on identical worksheets with minute details of birth circumstances that foretell ICC or haphazard DCC. They also document the neonatal outcomes of cord clamping, ICC or DCC. These worksheets are sent in to State Departments of Community Health for computer scanning and recording of all data.

Birth Certificate Statistics
Compilation of birth certificate statistics across the United States is relatively uniform, and apart from time, date, place and family identities, a wealth of impartial birth data (Figures 11,12) is recorded. Maternal outcome (e.g. C-sect v. vaginal birth, attendant – MD, midwife, other etc.) and newborn outcome (e.g. Apgar score, admission to NICU) are recorded in State health departments and are available for computerized analysis. The birth certificate data that relate to BBI, CP and NE are abnormalities of the newborn that correlate with the signs and symptoms of and neonatal encephalopathy. [4]
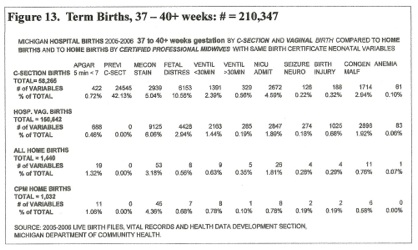
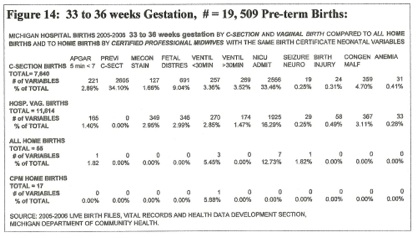
The time of cord clamping after birth is not individually recorded; however, the type and location of the delivery indicates a probable and definite (home CPM) range for the time of cord clamping and the corresponding amount of PT. The following statistics are from all live births in the State of Michigan during 2005 and 2006 totaling 235,215 live births. [Figures 13, 14, 15]
Cesarean sections will usually have had ICC or early cord clamping as ICC and removal to resuscitation is the standard-of-care protocol for C-S / “at risk” births in most hospitals. Elective C-sections (ECS) – no labor – will have minimal or negative PT. C-Sections in labor may receive some PT depending on clamp timing (figure 5.)

Vaginal “at risk” deliveries in hospital will have had ICC (fetal distress) when an NICU team is present, but some / much of the placental transfusion may be delivered in 20 seconds before a clamp can be applied. (Figure 3.) “Normal” vaginal deliveries may have DCC, and PT is likely to have been at least partly accomplished at any vaginal birth as the mother pushes out the child together with some placental blood. [Figure 6.] Some obstetricians / midwives wait for the baby to breathe or cry at vaginal delivery before clamping – almost maximal PT.
Home deliveries are done mainly by Certified Professional Midwives who routinely do not clamp the cord until the placenta has delivered. The home, CPM delivered child routinely has an optimal, maximal, physiological blood volume. Other home deliveries, many accidental, may be similarly advantaged, especially if no cord tie is available.
There are thus three main birth modes recorded on birth certificates that relate to the timing of cord clamping and resultant placental transfusion, leading to three basic premises:
Cesarean births – ICC, overall – minimal PT, especially ECS.
Hospital Vaginal births – moderate transfusion (Some ICC for fetal distress, many inevitable partial transfusions, some full.)
Home births – CPM – maximal transfusion (CC after placental delivery.)
The condition of the neonate is recorded in great detail. The gestational age in weeks [figure 11] separates neonates into three categories:
>36 weeks gestation, (term.)
33 – 36 weeks (preterm.)
< 33 weeks (Premature.)
Further definition of these categories, listed as “Variables” [figures 13, 14, 15] indicates pathology of the neonate and abnormalities of the birth:
5 minute APGAR SCORE <7
Previous C-Section
Meconium staining
Fetal Distress
Ventilation for < 30 minutes Ventilation for > 30 minutes
Admission to NICU
Seizure or Neurologic dysfunction
Birth Injury / bone fracture, peripheral nerve injury
Congenital Malformations
Anemia
Analysis of the Variables, Figure 13: 37 to 40+ weeks gestation
The Apgar score: Color (red 2, blue 1, white 0) Breathing (++2, +1, -0.) Pulse rate (>100 = 2, <100 = 1, 0 = 0.) Tone (2, 1, 0) reflexes (2, 1, 0). These numbers assess some of the child’s life support organs and systems. The Five minute Apgar score below 7 (6 – 0) may be significant if the circumstances of the neonate at 5 minutes and method of scoring is understood. CPM’s and many others score blue as zero color. Apgar’s original zero color was WHITE. White indicates HYPOVOLEMIC SHOCK; pallor (any color) indicates a low blood volume. Blue / Red (generalized) indicates a good blood volume. The heart rate indicates oxygenation, >100 oxygenated, <100 hypoxic, 0 anoxic. [fig 10]
With NICU resuscitation, a 5 minute Apgar 6 is ominous – by 5 minutes the lungs are ventilated, either spontaneously or by intubation (breathing 2, color 2, heart rate 2) and the four missing Apgar points are usually missing from CNS indicators – tone and reflexes – matching Cowan’s 3 mandatory criteria for NE, [4] abnormal tone, no suckling reflex, lack of awareness.
In the Home birth 5 minute Apgar 6, with the cord pulsating, the child may be sleeping peacefully on the placental circulation, blue, (0) not breathing (0) – see Figure 4. At 5 minutes, Apgar 6, the child kicked the bed and at 9+ minutes started crying – not very ominous. A pulsating cord and a functioning placenta are the equivalent of functioning lungs, kidneys, liver and gut. The hospital 5 minute Apgar reflects the injuries caused by amputating a functioning placenta.
In hospital births, C-sections had nearly twice (0.72%) the incidence of Apgar <7 over vaginal births (0.46%). Home births had the highest incidence of 5min. Apgar<7, (1.06%) but third stage management was very different from hospital birth, functioning placentas were not amputated.
The Previous C-Section indicator shows that VBACS are rare, and that elective repeat C-sections are the rule, (42% of all C-sections). This may not be beneficial for the neonate.[33, 34, 35, 36]
Meconium Staining seems to have little influence on delivery mode or outcome, unless it loads the “fetal distress category” into cesarean delivery – 10.56% CS v. 2.94% vaginal birth. Again, this may not be beneficial for the neonate.[ 33, 34, 35, 36]
Fetal Distress in hospital apparently leads to many C-sections – with routine ICC and removal to resuscitation. In CPM home births, meconium staining is not mirrored in the fetal distress column, and possibly due to lack of
fetal monitoring, home births have very little fetal distress – that appears to be benign as there is very little NICU admission with Home birth. “Distressed” CPM home births are resuscitated by PT—as are all CPM births.
Ventilation for less than 30 minutes: This is an indication of minor respiratory distress (“Gasping” MOD) due to hypovolemia / hypotension and is highest (2.39%) in C-sections, (ICC, minimal PT) and lowest (0.78%) in Home births by CPM’s – Full PT. In Home births, ventilation probably indicates temporary use of a bag-mask only, not intubation.
Ventilation for More than 30 minutes: Major respiratory distress and MOF – these 329 CS neonates together with 285 vaginal births are probably “Sick” neonates that will have long-lasting neural and mental problems; some may have CP. These term neonates had adequate amounts of surfactant; their RR and RDS probably correlated with hypotension [fig. 1]
Note that only one Home CPM birth, 0.10%, is included and would have been admitted to NICU from home. This case may have had a tracheo-esophageal fistula in the congenital anomalies group, or possibly meconium aspiration; whatever the diagnosis, this child received a full placental transfusion and would not be hypotensive or have MOD. The total for 2005 – 2006 in this category (all gestation ages) is 1840 – 0.78% of 235, 215 – close to the 1% incidence of autism.
NICU Admission: The C-Section child [33, 34, 35, 36] is 2.3 times more likely to need intensive care (4.59% v 1.89%) than the vaginal birth child, and six (6) times more likely to need intensive care (4.59% v 0.78%) than the CPM Home birth child. The 8 Home birth NICU admissions could have been congenital malformations and birth injuries, complications unrelated to cord closure.
Seizures and Neurological Disorders: All groups record a very similar (and very low) percentage of seizures and neurological dysfunctions. This may be due to early recording of the birth certificate data that does not record the ultimate (late) neurological outcome of CP. Some “seizures” may be hypoglycemic. Cowan [4] separated seizures from the criteria for NE and did not project long-term outcome.
Birth Injury – Bone Fracture and Peripheral Nerve Injury: This category mainly addresses shoulder dystocia, clavicle and humerus fractures, and brachial plexus injury. The outcome of shoulder dystocia can involve generalized brain damage. [39] Note that c-section does not eliminate bone and nerve trauma, and that CPM management (home birth / no head traction / “Squatting, All Fours birth) produces minimal injury. ACOG management [39] includes “gentle” head traction to “test” the shoulder / brachial plexus. This maneuver can occur at C-section. C-sections for breech can involve nuchal arms and fractured humeri.
Congenital Malformations: 4629 neonates were “malformed” out of a total of 210,347 neonates over 37 weeks gestation. The list on the birth certificate worksheet has 18 numbered diagnoses to define these malformations. Late clamping should benefit these already compromised neonates. The higher percentage of hospital births in this category probably results from prenatal ultrasound diagnosis.
Anemia: Absence of “anemia” or presence of normal hemoglobin (Hg) on the birth certificate is NOT a guarantee of a normal neonatal blood volume. Hypovolemia may accompany a normal or high Hg. The Hg level falls gradually as the hypovolemia is corrected. Hypovolemia becomes immediately evident in terms of multi-organ dysfunction / failure—hypotension, oliguria, RDS, “slow to respond,” hypothermia, hypoglycemia etc.
As anemia is usually a late neonatal development, many “mildly” hypovolemic neonates are discharged before hemo-dilution occurs or before hemoglobin is drawn. If “anemia” is noted on a birth certificate worksheet it indicates considerable birth blood loss, an NICU stay while anemia develops, and probable transfusion of red cells [1] – a “sick” baby. For term C-section births, the incidence of “anemia” is one per thousand c-sections, approximating the incidence of cerebral palsy.
The 1032 home CPM term births that had
full placental transfusion incurred no anemia – no very “sick” neonates.
Figure 14: 33 to 36 weeks Gestation
Only 17 of the 55 home births were delivered by CPM’s, the rest (38) were delivered by a variety of attendants designated as: ”other 13,” “no attendant 4,” “husband, 7” “MD, 5” “nurse midwife, 1” “unknown, 8.” The CPM deliveries were without complication apart from one child that was bag-masked briefly; all received a full placental transfusion. [40, 41] These neonates had no surfactant in their lungs, indicating that the presence or absence of surfactant has little effect on RDS.
The other 38 home deliveries probably had delayed clamping (only 6 had professional care and probably avoided ICC and immediate ventilation.)
The incidence of anemia (very “sick” neonates) in this pre-term group is four times higher than the term group. (0.41% v. 0.10% CS, 0.28% v. 0.06% Vag.) The reason for this is probably the blood volume distribution between placenta and fetus — the more premature the birth, the more blood is in the placenta; ICC clamps a larger fraction of blood volume in the placenta in the pre-term child. This trend is accentuated in the premature <33 weeks group.
The benefit of full placental transfusion is obvious in the 17 CPM home births (no anemia, no complications.) [40, 41] None of the home births developed anemia.
Of the hospital births, most “sickness” indicators showed predominance in the c-section deliveries – vent < 30 min. 3.4%, Vent > 30 min. 3.5%, and a very high NICU admission rate – 33% compared to a Home CPM NICU admission rate of ZERO! Placental transfusion for the pre-term child is a necessity [40, 41]
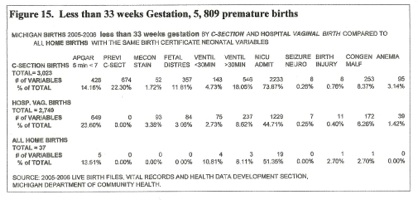
Figure 15: Less than 33 weeks Gestation
Cesarean births exceeded vaginal births, but 5 minute Apgar scores below 7 were higher in the vaginal birth group. Percentage of long term ventilation was highest in the c-section group. NICU admission figures were probably not relevant as some rural hospitals do not have an NICU, and use the regular newborn nursery. The 37 home deliveries had only one professional attendant and probably reflect emergency room assessment on admission after a “natural” birth. Percentages for variables for home and hospital vaginal birth show little difference in “variables.”
The anemia factor is significant. None of the home births became anemic despite 51% of them being admitted to the NICU. Presumably, they had “blood draws” for continued NICU evaluation that are said to contribute to neonatal “anemia.” None were delivered by CPM’s, but very few would have a cord tie or clamp available during the moments after birth; most, by accident, received a full PT. Overall, CS (little PT) produces much sicker preemies than vaginal delivery (some PT). [17]
The Effects of the Three Delivery Modes on Newborn Outcome
A good indicator of lack of PT / hypovolemia / ischemia is the degree of a neonate’s “sickness,” the SNAP II score; [Score of Acute Neonatal Physiology.] [23] Sick neonates become anemic and usually need red cell blood transfusions. [1] While SNAP II scores are not recorded on the birth certificate, many indicators of neonatal “sickness” are recorded. These are MOD’s. The incidence of these Birth Certificate “sickness indicators” should reflect the amount of blood loss in each mode of delivery.
Several of the above eleven “variables” could have been obtained from an individual birth certificate, e.g. fetal distress, 5 min. Agar <7, ventilation >30 mins., and NICU admission, could all come from one birth certificate. Thus the total of listed “variables” is higher than the total number of neonates that were affected by those variables. One neonate could have several “variables” and many neonates would have none. However, this “exaggeration” of the incidence of pathology applies equally to each mode of birth – C-section, Hospital Vaginal, and Home birth, and should not affect the relative injurious potential related to the original premises regarding the time of cord clamping for each mode, and the amount of PT delivered in each mode.
37 – 40+ Weeks Gestation
In Figure 13, the “variables” relating to “sick” neonatal outcome are Apgar<7, Vent <30 min, Vent > 30min, NICU Admit, Seizure, Birth Injury. In all except Birth Injury, Cesarean section results in a higher percentage of “sick” neonates than does hospital vaginal birth. Some of this “shift to sickness” may be attributed to an overload (10.6% v. 2.94%) of fetal distress cases (Cowan’s NE late decelerations, meconium staining) in the C-section mode – the “at risk” fetus (possibly hypovolemic) is moved from labor to surgery (not necessarily to safety). At birth, vaginal or CS, “Fetal distress” is routinely clamped immediately and moved to resuscitation, moving both modes of delivery towards “sickness,” (no PT) but with an excess number in the CS mode. However, with vaginal birth, intra-uterine and abdominal pressure may force in some PT before a fast clamp can be applied.
On the other hand, the C-section mode is overloaded with the “Previous CS” variable (42.13% repeat CS v. 0.00% VBAC.) Many, if not most of these elective “repeat sections” (ECS) are “risk free,” term, scheduled, operative deliveries that bypass the intra-partum complications of labor and vaginal birth; they should be free from “sick” outcomes and should more than compensate for the excess toward “sickness” in “fetal distress” C-sections. Despite this excess of “managed safety,” the C-section neonate’s risk of NICU admission and the risk of needing ventilation are twice that of vaginal birth. ECS births are routinely subjected to ICC, and in the absence of labor, the PT does not initiate pulmonary blood low and pulmonary ventilation. [14] Persistent fetal circulation and neonatal demise are uncommon “untoward outcomes.” [34, 35, 36]. Overall, “safe” elective C-sections make cesarean birth much less safe for the neonate than vaginal birth in hospitals. The basic premises that cesarean births result in minimal PT compared to vaginal births that result in moderate PT appear to be validated by these outcomes.
Any of these “sickness indicators” that resulted in resuscitation in hospital will increase the risk of childhood mental retardation. [38]
The 1,032 Home CPM neonates on Figure 13 routinely received a full PT and cords were clamped after the placenta delivered. As mentioned previously, the high 5 min. Apgar <7 percentage does not indicate “sickness” as a number of these CPM newborns would still have functioning placentas after 5 minutes. The incidence of NICU admission and ventilation support was half that of vaginal hospital birth and one quarter that of CS birth. There were only 8 admissions to NICU; all these babies were resuscitated by PT and it is unlikely that they had respiratory distress; NICU admission may have been for congenital anomalies, birth injury or meconium aspiration, conditions not influenced by the time of cord clamping. This “mode” is the only mode that has unobstructed physiology operating; the obvious outcome is health. None of these neonates were subjected to “resuscitation.” None were at risk for mental retardation. [38]
33 – 36 Weeks Gestation
In Figure 14, the CS vs. Vag birth rate is 39%, and 34% of those sections are “repeat.” However, it is unlikely that these were “elective” preterm sections, most were probably in labor; “fetal distress” sections outnumber vaginal fetal distress births 9% to 3%. The “variables” relating to “sick” neonatal outcome are Apgar<7, Vent <30 min, Vent > 30min, NICU Admit, Seizure, Birth Injury. The percentage of “sick” neonates produced by CS was roughly twice that from vaginal birth; however the number of “sick” CS neonates was less than one third the number admitted to the NICU. Respiratory distress was the major “sickness.”
The percentage of “sickness” variables for vaginal births in this gestational age group was generally higher (more than double) those for term neonates [3740+] and the NICU admission percentage was much higher – 16.29% vs. 1.89%. An implicating factor for this increase in “sickness” may be the mandatory presence of an NICU team at preterm births with a protocol of mandatory ICC and removal to a resuscitation table – no PT. One would expect that this excess of “resuscitation” would result in excess mental retardation in preterm babies. [38, 30]
In the 17 CPM Home births, the presence of a full PT and the absence of an NICU team (no resuscitation [38]) produce a striking absence of “sickness,” – total health. [41]
Less than 33 Weeks Gestation – Premature Births:
In the premature group, <33 weeks gestation, the incidence of pathology and “sickness” predominated in the C-Section births. There were no home CPM deliveries in this group, but the 37 home births, judged by “sickness” indicators, were as healthy as hospital vaginal births. None of the home births became anemic in the NICU, indicating the value of the absence of a cord clamp in the home, and the absence of a resuscitation team. [38]
Discussion
During the third stage of labor, the child goes through a transition from maternal / placental life support to atmospheric living on all the child’s life support organs and systems. Placental function is reflexively terminated after the independent life support systems are reflexively initiated.
This article is founded on a single premise: That the third stage of labor (from the birth of the child to the delivery of the placenta) is a complex physiological (totally healthy) event. Complexity increases the possibility of accident and harm. However, if any part of this event were inherently harmful, survival at birth would be compromised, and the innate defect would eventually be “bred out.” Birth physiology is perfected health.
External disruption of any part of the third stage is injurious – it disrupts health. Major misconceptions regarding third stage events are:
That the placental transfusion is harmful, causing plethora, (too much blood) polycythemia and hyperviscosity, and that hypoxia causes brain damage.
These fallacies have led to birth management that clamps the cord to avoid imaginary illness, and amputates placental oxygenation to facilitate pulmonary oxygenation. Despite an over-abundance of information (Cochrane reviews, databases etc) on the timing of cord clamping, perinatal science authorities cannot answer the question: “When Should We Clamp the Umbilical Cord?” [42]
“… The optimal timing for this intervention remains controversial.” [13] This question and its resulting enigma are founded on a basic fallacy – that the cord SHOULD be clamped; Erasmus Darwin gave mandates on when the cord SHOULD NOT be clamped. [5] His grandson elaborated by explaining that Nature (physiology) clamps the cord when the newborn has achieved optimal fitness to survive; otherwise, mammals would be extinct. Optimal health at birth is achieved without a cord clamp. [41]
Evaluation of the Injuries
The “MOST IMPORTANT FINDING [13, 32] was that DCC resulted in a 47% reduction of risk of infant anemia and a 33% reduction of risk of having deficient iron stores at 2 to 3 months” and that this “benefit” extends into infancy. [32] The authors [13, 32] do not elaborate on the “important benefit” of preventing infant anemia – the PREVENTION OF MENTAL RETARDATION. Lozoff’s research [28, 29, 30] (with 99 references) is not included in the references of these cord clamping articles, but Van Rheenen’s article [43] is, and he refers to: “victims of immediate clamping …” “Infant anemia is associated with impaired mental and motor development.”
“At 5 years, each 10 g/L increment in Hb [taken at 9 months] was associated with a 1.75 point higher IQ score.” [29] How many obstetricians understand that when a pulsating cord is clamped, and enough blood volume is removed to cause anemia in infancy, the child’s IQ will be lowered for the rest of its life, possibly to the extent of perpetual dependency? [24]
Neonatal blood volume “sufficiency” is measured by how well the heart pumps blood through the neonatal vital organs; the heart’s vital “signs” are blood pressure and central venous pressure – cardiac output and cardiac filling. Neonatal blood volume “sufficiency / insufficiency” is reflected in vital organs:
Kidneys – urine output, diuresis / oliguria
Lungs – regular breathing / respiratory distress, gasping
Gut & liver – active suckling / lethargy, hypoglycemia
Skin – warmth and plethora / pallor and hypothermia
Brain and CNS – Vigorous action / loss of tone, reflexes, loss of response.
The introductory portion of this paper addresses mainly anatomical diagrams, mechanical recordings of anatomical changes, and laboratory values (e.g cord pH) that are finite, and may be indicative of previous and / or continuing function, e.g. the “stepwise” increase in weight indicating reflexive control of blood flow into the neonate, or the effect of gasping” on diastolic blood pressure and heart rate in the asphyxiated primate. (Figure 1.) They are dissected portions of anatomy [14] and physiology that help to explain total form and function in the intact individual – neonate, cord and placenta.
The “Birth certificate” portion of this study is an analysis of the dispassionate recordings of neonates’ “abnormalities and problems” correlated with three types of birthing events that generate, on average, differing amounts of PT:
1. Cesarean section, minimal PT
2. Vaginal hospital birth, moderate PT
3. Home birth, by CPM, Maximal PT.
The influence of the amount of placental transfusion on the health of the neonate becomes readily apparent by the number of abnormalities and problems generated in each birth type.
The effect of the amount of PT on newborn morbidity is very evident. The incidence of problems in Cesarean births is more than double that in hospital vaginal births, while serious abnormalities become rare in in CPM home births. This discrepancy becomes wider in pre-term and premature births where neonatal resuscitation with ICC, (and little or no PT) is protocol.
The “abnormalities and problems” selected from birth worksheets are those that would indicate blood loss and possible signs of NE. Detailed statistical analysis should not be needed to reach a verdict on hospital third stage management.
The actual incidence of brain injury in these children must await neurological and mental evaluation in later years, but using anemia and NE “sickness” predictors on the birth certificate worksheet, few, if any of the CPM home births should have neural, mental or behavioral defects in childhood or grade school. This optimistic forecast cannot be made for the “cord-clamped” cohort of 234,166 children. This survey was completely anonymous, so individual follow up is impossible.
Based on past experience, about 1 per thousand of these children (234) will have some degree of cerebral palsy, and about one in 100 (the current incidence of “autism”) will be placed in SE ~ (2,342). These SE children, diagnosed by IQ evaluation, will be recorded by the State Department of Community Health, and their birth certificates are available for computerized analysis. Validation of these SE – Birth Certificate statistics, correlating IQ with birth cord clamp injury should be available by 2012 – 2015.
The remainder of the “cord clamped” cohort, ~ (230,000) cannot be presumed to be “normal,” if the “NORM” is the physiological – that category is reserved for Home CPM births. Many of these “remainder” children were deprived of some blood volume. Minimal brain damage may have reduced the IQ from a high potential to low average, but not low enough to place the child in SE. This reduction in cognitive ability and increase in ASD features would be much more apparent in boys than in girls, especially when scholastic achievement is evaluated. Today, this sexual imbalance is very evident in all educational establishments:
Edited from the WSJ 2006 (Where the Boys Aren’t By Christina Hoff Sommers [44] ): Vast numbers of high school boys are doing poorly; more boys than girls drop out. The number of boys diagnosed with disabilities has exploded in the past 30 years. High school boys’ achievement is declining in most subjects.
It is true that our colleges are now 57% female. The reading scores of 17-year-old boys overall have gone down in the past decade, hitting an all-time low in 2004. Reading skills: 23% of the white sons of college educated parents scored “below basic.” For girls from the same background, the figure is 7%. One in four boys who have college educated parents cannot read a newspaper with understanding.”
Today, for every 100 women who earn a bachelor’s degree, just 73 men get one.
Similar figures are available from armed forces recruitment centers – 70% of high school graduate applicants fail 8th grade math and English tests. The teaching profession may be partially at fault, but not entirely. The male / female discrepancy is difficult to explain otherwise.
This shift has occurred over the past 20 to 30 years and has paralleled the “autism epidemic” of retarded children (more boys than girls) being placed in SE. It also coincides with a Cesarean Section epidemic, (CS was about 5% of live births in 1960’s to CS 35+% of live births today.) The practice of instant resuscitation by ICC and ventilation started in the early 1980’s and is now universal for “at risk” births. The consequences of these perinatal practices are documented on birth certificate worksheets. The ultimate consequences are documented on SE admissions and student S.A.T. scores.
Validation of the above postulations can be rapidly achieved in any large obstetrical institution by permitting physiological cord closure at every birth, term, pre-term and premature, and by not clamping and cutting the cord until the placenta has been expelled from the uterus at every birth. Within two weeks, the NICU will be virtually empty. Validation of the SE issue will take 6 or 7 years, and the S.A.T. issue will not be apparent for 16+ years.
Summary and Conclusions
Current hospital perinatal management of the third stage of labor is injurious. After the child is delivered, the umbilical cord should be left intact until the placenta has been delivered. Maneuvers to maintain the integrity of the feto-placental / neonatal-placental circulation should be taken (Somersault maneuver. [21]) Resuscitation of the compromised neonate should be based on the use of a functioning placenta and a pulsating umbilical cord. Neonatal resuscitation measures should be limited to establishing the newborn’s airway; iatrogenic pulmonary ventilation should be avoided. Spontaneous initiation of respiration can be enhanced by lowering the neonate well below the placenta, or by inducing contraction of the uterus around the placenta with intravenous oxytocin. These measures effect and enhance placental transfusion of blood into the neonate – erecting, expanding and ventilating the lungs and establishing function in all the neonate’s life support organs. After respiration is fully established, after all pulsation in the cord has ceased, and after the placenta is delivered into the atmosphere, the cord may be clamped and cut.
Abbreviations: Cerebral palsy CP, Birth brain injury BBI, Neonatal encephalopathy NE, Hypoxic Ischemic Encephalopathy HIE, Immediate cord clamping ICC, Delayed cord clamping DCC, Blood pressure BP, Placental transfusion PT, Respiratory Distress Syndrome. RDS, Retraction Respiration RR, Central Venous Pressure CVP, Central Nervous System CNS, Multi Organ Failure MOF, Multi Organ Dysfunction MOD, Germinal Matrix GM, Intra Ventricular Hemorrhage IVH, Fetal Heart Rate FHR, Elective Cesarean Section ECS, prostaglandin PG, Neonatal Intensive Care Unit NICU, Intelligence Quotient, IQ
Acknowledgements:
All of the birth certificate data were compiled from: 2005-2006 LIVE BIRTH FILES, VITAL RECORDS AND HEALTH DATA DEVELOPMENT SECTION, MICHIGAN DEPARTMENT OF COMMUNITY HEALTH.
About the Author: George Malcolm Morley, MD, FACOG is a practicing physician and medical researcher in the area of neonatal care. Please address the author at 10252 E. Johnson Road, Northport, MI 49670. E-mail: obgmmorley@aolcom.
References
1) Murray NA, Roberts AG. Neonatal transfusion practice. Archives of Disease in Childhood Fetal and Neonatal Edition 2004;89:F101
2) Windle WF. Brain damage by asphyxia at birth. Scientific American, 1969; 221(4):76–84.
3) ACOG Committee on Obstetric Practice Committee Opinion Number 348 November 2006; OBSTETRICS & GYNECOLOGY, Vol. 108, No. 5, November 2006.
4) Frances Cowan et al. Origin and Timing of Brain Lesions in Term Infants with Neonatal Encephalopathy. The Lancet, Vol. 361, Issue 9359,1 March, 2003 pages 736-742
5) Darwin E. (1801) Zoonomia, 3rd edition. London: vol III page 302. “Another thing very injurious to the child, is the tying and cutting of the navel string too soon; which should always be left till the child has not only repeatedly breathed but till all pulsation in the cord ceases. As otherwise the child is much weaker than it ought to be, a portion of the blood being left in the placenta, which ought to have been in the child.”
6) Peltonen T. Placental Transfusion, Advantage – Disadvantage. Eur J Pediatr. 1981;137:141-146
7) Linderkamp O. Placental transfusion: determinants and effects. Clinics in Perinatology 1982;9:559-592
8) Shah P, Riphagen S, Beyene J, Perlman M. Multiorgan dysfunction in infants with post-asphyxial hypoxic-ischaemic encephalopathy. Arch Dis Child Fetal Neonatal Ed 2004;89:F152-F155.
9) Hankins GDV, Koeh S, Gei AF. Neonatal Organ System Injury in Acute Birth Asphyxia Sufficient to Result in Neonatal Encephalopathy. OBSTETRICS & GYNECOLOGY; Vol. 99, Part 1, May 2002; 688-691
10) Myers RE. Two patterns of perinatal brain damage and their conditions of occurrence. American Journal of Obstet Gynecol, 1972; 112:246–76
11) Gunther M. The transfer of blood between the baby and the placenta in the minutes after birth. Lancet 1957;I:1277-1280.
12) Jose Luis Diaz-Rossello Facts and Ethics International Perspectives: Cord Clamping for Stem Cell Donation: NeoReviews 2006;7;e557-e563 DOI: 10.1542/neo.7-11-e557
13) Hutton EK, Hassan ES. Late vs Early Clamping of the Umbilical Cord in Full-term Neonates. JAMA, March 21, 2007—Vol 297, No. 11 1241-1252
14) Jaykka S, Capillary Erection and Lung Expansion, Acta Paediatr. Scand. 1965 [Suppl] 109.
15) J C McGrath, S J MacLennan, A C Mann, K Stuart-Smith. Contraction of human umbilical artery, but not vein, by oxygen. J. Physiol. 1986;380;513-519
16) Kinmond S, Aitchison TC, Holland BM, Jones JG, Turner TL, Wardrop CA. Umbilical cord clamping and preterm Infants: a randomized trial. BMJ, 1993 Jan 16;306(6871):172–5
17) The Cochrane Report: Review of Delayed Umbilical Cord Clamping In Preterm Infants.
18) Mercer J, McGrath M et al, Immediate and Delayed Cord Clamping in Infants Born Between 24 and 32 weeks. Journal of Perinatology, 2003; 00: 1 – 7.
19) Mercer j, Nelson CC, Umbilical cord clamping: Beliefs and practices of American nurse midwives. J. Midwifery Women’s Health. 2000: 45: 58-66.
20) Cashmore J, Usher RH. Hypovolemia Resulting from a Tight Nuchal Cord at Birth. Pediatric Res. 1973; 7:339.
21) Mercer JS, Skowgaard RL. Nuchal Cord Management and Nurse Midwifery Practice. Journal of Midwifery and Women’s Health; Vol. 50, No. 5: Sept Oct 2006, 373-378.
22) LETTERS TO : Shah P, Riphagen S, Beyene J, Perlman M. Multiorgan dysfunction in infants with post-asphyxial hypoxic-ischaemic encephalopathy. Arch Dis Child Fetal Neonatal Ed 2004;89:F152-F155. GOOGLE: Arch Dis Child Fetal Neonatal Ed 2004 SHAH LETTERS MORLEY
23) Limperopoulos C, Bassan H. Positive Screening for Autism in Ex-preterm Infants: Prevalence and Risk Factors. DOI: 10.1542/peds.2007-2158. Pediatrics 2008;121; 758-765
24) Hack M, et al., Outcomes in Young Adulthood for Very Low Birth-weight Infants. New Engl J Med, Vol. 346, No.3, Jan, 2002:149-157
25) Hultman CM, Sparen P, Cnattingius S. Perinatal risk factors for infantile autism. Epidemiology, 2002 Jul;13(4):417–23.
26) Matsuishi T, Yamashita Y, Ohtani Y, Ornitz E, Kuriya N, Murakami Y, Fukuda S, Hashimoto T, Yamashita F. Brief report: incidence of and risk factors for autistic disorder in neonatal intensive care unit survivors. J Autism Dev Disord, 1999 Apr;29(2):161–6
27) Bolton PF, Murphy M, Macdonald H, Whitlock B, Pickles A, Rutter M. Obstetric complications in autism: consequences or causes of the condition? J Am Acad Child Adolesc Psychiatry, 1997 Feb;36(2):272–81
28) Lozoff B, Beard J, Long-lasting neural and behavioral effects of iron deficiency in infancy Nut. Rev 2006 May 64 (5 PT 2): S34-S43
29) Palti H, Meijer A. Learning achievement and behavior at school of anemic and non-anemic infants. Early Human Dev. 1985; 10:217-223
30) Hurtado EK et al. Early childhood anemia and mild to moderate mental retardation. Am J Clin Nut. 1999; 69(1): 115-9.
31) Allen LH. Anemia and Iron Deficiency: effects on pregnancy outcome. Am. J. Clin. Nutr. 2000; 71: 1280S-1284S.
32) Chaparro CN, Neufels LM. Effect of timing of umbilical cord clamping on iron status in Mexican infants: a randomized trial. The Lancet; Vol. 367 June 17, 2006.
33) Glasson EJ, Bower C, Petterson B, de Klerk N, Chaney G, Hallmeyer JF, Perinatal factors and the development of autism: A population study. Arch, Gen. Psychiatry; 2004 JUN 61; (6) 618-27.
34) Levine et al. Mode of Delivery and Risk of Respiratory Diseases in Newborns. OBSTETRICS & GYNECOLOGY, 2001; 97: 439-42
35) Morley GM. LETTERS OBSTETRICS & GYNECOLOGY, Vol 97, No.6,June 2001, 1024-1026
36) Anne Kirkeby Hansen, Kirsten Wisborg. Risk of respiratory morbidity in term infants delivered by elective caesarean section: cohort study. doi:10.1136/bmj.39405.539282.BE BMJ 2008;336;85-87; originally published online 11 Dec 2007;
37) Sugie Y, Sugie H. Neonatal Factors in Children with Autistic Disorder and Typically Developing Infants. Autism 2005; Sage Publications vol. 9(5)487-494
38) Odd DE, Lewis G, Whitelaw A, Gunnell D. Resuscitation at birth and cognition at 8 years of age: a cohort study. www.thelancet.com. Published online April 21, 2009 DOI:10.1016/S0140-6736(09)60244-0
39) ACOG PRACTICE BULLETIN, Number 40. November 2002. Shoulder Dystocia
40) Kees Ultee, Joost Swart, Hans van der Deure, Carole Lasham and Anneloes van Baar Delayed cord clamping in preterm infants delivered at 34 to 36 weeks gestation: A randomized controlled trial. Arch. Dis. Child. Fetal Neonatal Ed. published online 16 Feb 2007;
41) Morley GM. LETTER Ultee The optimal time to clamp the umbilical cord. Arch Dis Child Fetal Neonatal Ed 2007;000:1. doi:10.1136/adc.2007.119115
42) Philip A, Saigal s. WHEN SHOULD WE CLAMP THE CORD? e142 NeoReviews Vol.5 No.4 April 2004
43) Patrick F van Rheenen, Bernard J Brabin. A practical approach to timing cord clamping in resource poor settings. BMJ 2006;333:954-958 (4 November), doi:10.1136/bmj. 39002.389236.BE
44) Wall Street Journal, July 3, 2006 Ms. Sommers is the author of “The War Against Boys” (Simon & Schuster, 2000). ]
45) Sechzer JA, Faro MD, Barker JN, Barsky D, Gutierrez S, Windle WF. Development behaviors: delayed appearance in monkeys asphyxiated at birth. Science. 1971 Mar 19;171(976):1173–5.
46) Sechzer JA, Faro MD, Windle WF. Studies of monkeys asphyxiated at birth: implications for minimal cerebral dysfunction. Semin Psychiatry. 1973 Feb;5(1):19–34.